When you suspect an invasive fungal infection (IFI), one day can be the difference between life and death.*
With a robust fungal menu, differential testing, faster result turnaround time and diagnostics to monitor drug levels, Eurofins Viracor can help you better support your patients' continuum of care — from the onset of symptoms to managing dosage.
* Fungal tests result the same day (within 8-12 hours) of specimen receipt.

Testing to individualize your approach for invasive fungal infections
When you suspect your immunocompromised or transplant patient has an invasive fungal infection (IFI), one day can be the difference between life and death.
With a robust fungal menu, differential testing, faster result turnaround time and diagnostics to monitor drug levels, Eurofins Viracor can help you better support your patients' continuum of care — from the onset of symptoms to managing dosage.
When Timing is Critical,
Can You Afford Not to Know?
Risks of Treatment Delay
Treat IFIs Faster, Reduce Mortality
Managing fungal infections in immunocompromised individuals can be challenging due to a lack of specific symptoms and increased drug resistance. A rapid and accurate diagnosis is key in reducing the risk of mortality due to delayed treatment.1,2

Utilizing Therapeutic Drug Monitoring
Manage Drug Levels, Keep Patients in the Therapeutic Range
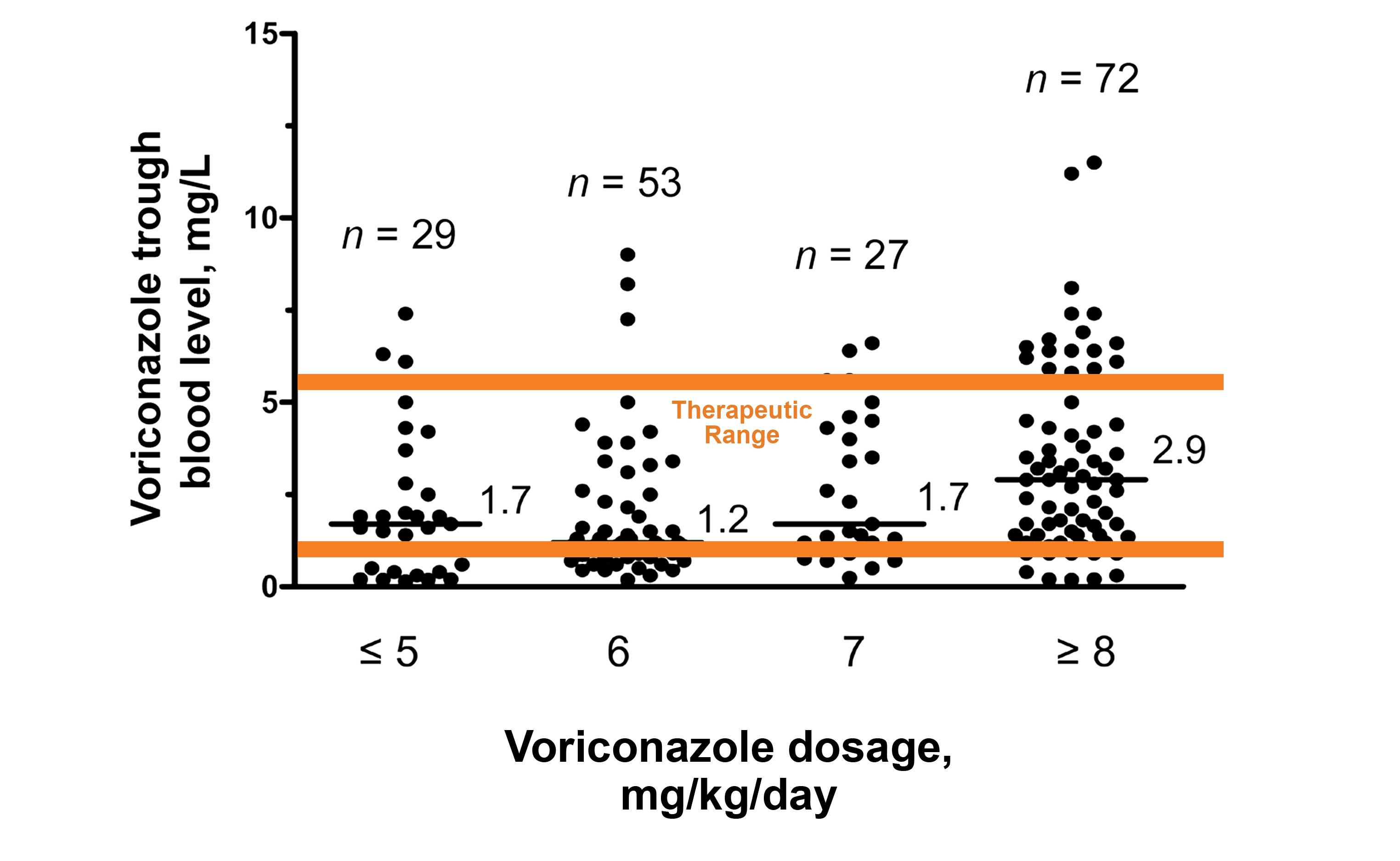
For high-risk IFI patients, early individualized treatment is critical. Antifungal therapeutic drug monitoring is necessary for managing efficacy and safety.
The below chart shows the relationship between voriconazole dosage and blood levels.
In this study, up to 35% of patients samples were outside the therapeutic range.3
Diagnostics for Fungal Patients' Complete Continuum of Care
With testing that aids in differential diagnosis, faster treatment and drug level monitoring, your patient will do better and the hospital can lower costs.
Depend on Eurofins Viracor for clinical testing and rapid turnaround time that make a difference for everyone.

Explore our Fungal Test Menu
Test Name |
Test Code |
Turnaround Time |
| Fungal Plus PCR Profile I Aspergillus • Nocardia • Mucorales |
PFL8005 |
Same day (within 12 - 24 hours from receipt of specimen), Monday through Friday. |
| Fungal Plus PCR Profile II Aspergillus • Nocardia • Mucorales • Pneumocystis jiroveci |
PFL8006 |
|
| Fungitell® β-D-Glucan |
1700 |
|
| Fungitell® β-D-Glucan with Reflex to Titer |
351700 |
Same day (within 8-12 hours from receipt of specimen), Monday through Saturday. Additional 8-24 hours when reflex testing is required. |
| Aspergillus Real-time PCR Panel |
8900 |
|
| Candida Real-time PCR Panel |
33306 |
Same day (within 12 - 24 hours from receipt of specimen), Monday through Friday. |
| Candida auris Real-time PCR (axilla/groin or nares) |
33330 |
Same day (within 12 - 24 hours from receipt of specimen), Monday through Friday. |
| OMEGA Coccidioides Antibody EIA |
3400 |
3 days from sample receipt. Testing performed at VRL-Eurofins. |
| Aspergillus Galactomannan EIA |
1600 |
Same day (within 8 - 12 hours from receipt of specimen), Monday through Saturday. |
| Histoplasma Galactomannan EIA |
30881/30882/33222/33251 |
Same day (within 8 - 18 hours from receipt of specimen), Monday through Saturday. |
| Mucorales Real-time PCR |
3200 |
Same day (within 12 - 24 hours from receipt of specimen), Monday through Friday. |
| Nocardia Real-time PCR |
6800 |
Same day (within 8-12 hours from receipt of specimen), Monday through Saturday. |
| Pneumocystis jiroveci Quantitative Real-time PCR |
2000 |
|
| Isavuconazole (CRESEMBA®) LC-MS/MS |
4900 |
|
| Itraconazole LC-MS/MS |
2800 |
|
| Posaconazole LC-MS/MS |
4200 |
|
| Voriconazole LC-MS/MS |
3300 |
|
| Blastomyces qPCR (Lower respiratory) & Blastomyces qPCR (CSF) |
33469/33521 |
Same day (< 24 hours from receipt of specimen), Monday through Saturday. |
| Coccidioides qPCR (Lower respiratory) & Coccidioides qPCR (CSF) |
33470/33523 |
Same day (< 24 hours from receipt of specimen), Monday through Saturday. |
| Cryptococcus qPCR (Lower respiratory) & Cryptococcus qPCR (CSF) |
33471/33524 |
Same day (< 24 hours from receipt of specimen), Monday through Saturday. |
| Histoplasma qPCR (Lower respiratory) & Histoplasma qPCR (CSF) |
33468/33519 |
Same day (< 24 hours from receipt of specimen), Monday through Saturday. |
| NeXGenTM Fungal / AFB NGS (WB/Plasma) Assay |
33356 |
3 business days |
| NeXGenTM Fungal / AFB NGS (BAL) Assay |
33457 |
3 business days |
Fungal Posters
Mucor
Invasive Mucormycosis Management: Mucorales PCR Provides Important, Novel Diagnostic Information
In immunocompromised patients, high mortality and morbidity of invasive mucormycosis (IM) remain significant healthcare issues due in part to confusion of IM with invasive aspergillosis (IA) and failure to initiate appropriate therapy. A validated Mucorales (MUC) PCR detects the causative agents of IM with good sensitivity and specificity, as reported previously (M-227, ICAAC 2013). Published studies have not definitively determined the frequency of patients for whom pulmonary IA is suspected but IM is present. We aimed to (1) estimate the frequency of MUC PCR positivity in bronchoalveolar lavage (BAL) samples submitted for Aspergillus (ASP) PCR panel testing.
Nocardia
Addressing Pulmonary Nocardiosis Risk In Immunocompromised Patients: Development and Validation of a Commercially Available PCR
Pulmonary nocardiosis is an infection targeting immunocompromised patients characterized by high mortality and requires non-frontline antibiotics for treatment. Nocardiosis is currently confirmed or excluded by BAL fluid culture followed by further phenotypic identification steps. A culture-independent method with more timely results would accelerate the administration of appropriate treatment. A rapid Nocardia (NOC) PCR assay for BAL has neither been previously validated nor offered for clinical testing to our knowledge.
Aspergillus
A Reproducible, Scalable Method For Detection of Aspergillus From Formalin-Fixed Parafin Embedded (FFPE) Tissue Samples
Invasive fungal infections are a serious concern for immunocompromised patients. Aspergillus is a common fungal pathogen among both solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) that has a mortality rate of 35-59% in these populations2,3. Early diagnosis is important to determine the proper antifungal treatment and reduce mortality.
Let's Get You Started
Set Up An Account
We want to be your clinical lab partner.
When you work with us, you're accessing over 35 years of diagnostic innovation and specialized expertise in infectious disease, pre- and post-transplant, allergy and immunology testing for immunocompromised and critical patients.
Let's get started!
References
1 Assoc of Cape Cod, Fungitell Assay Instructions for Use. 2011 (Feb) Garey et al, Clin Infect Dis. 2006; 43:25-31. Karageorgopoulos et al. Clin Inf Dis. 2011; 52:750-770
2 Luong ML et al. Clin Inf Dis. 2012; 52 (10):1218-1226. Nguyen ML, Wissel MC et al. Clin Inf Dis. 2012; 54:1240-1248.
3 Andres Pascual, Thierry Calandra, Saskia Bolay, Thierry Buclin, Jacques Bille, and Oscar Marchetti, Voriconazole Therapeutic Drug Monitoring in Patients with Invasive Mycoses Improves Efficacy and Safety Outcomes, Clinical Infectious Diseases, 2008:46(2);204 by permission of Infectious Diseases Society of America.

